Digital Pharma: Janssen unveils UK social media plans
pharmafile | September 23, 2010 | News story | Medical Communications | Digital Pharma blog, Facebook, Janssen, Twitter, psoriasis, social media, youtube
Janssen UK is to launch a wide-ranging social media campaign to raise awareness of the chronic skin condition psoriasis.
The company’s digital strategy and social media manager Alex Butler unveiled the plans during InPharm’s digital marketing webcast yesterday.
The online event also saw presentations on the UK’s Code of Practice from Compliance Hub’s Steve Gray and on the digital environment, including healthcare professionals use of digital technology and online CME, from ICR UK and IMI’s Peter Mas-Mollinedo.
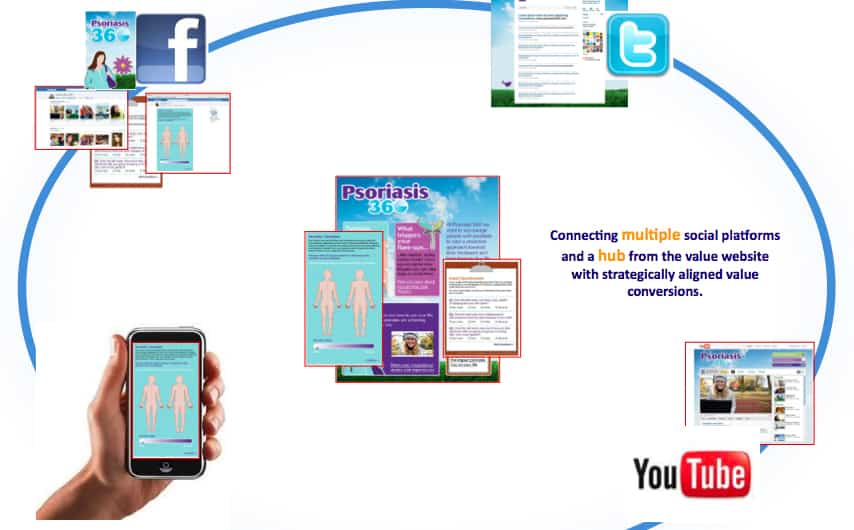
Janssen’s forthcoming Psoriasis 360 disease awareness campaign will encompass a Facebook page, Twitter account, iPhone and iPad apps and a YouTube channel, all centred around a main website.
The company has also recently updated its Psoriais iPhone app for healthcare professionals, adding a news feed.
Psoriasis, which results in the overproduction of skin cells, is notoriously difficult to treat and affects around 1.5 million people in the UK, up to 20-30% of whom suffer severely.
Janssen’s key product in the area is Stelara, a first-in-class biologic treatment, which was approved in Europe last year as a treatment for psoriasis.
Janssen’s social media charter
During the InPharm webcast Alex Butler also unveiled Janssen’s social media charter, a series of guiding principles that start by acknowledging the company has “a responsibility to our communities to encourage better health and education”.
The charter was clearly written with a close eye on the UK pharma’s self-regulatory Code of Practice and what it means for online activity and patient information.
Janssen’s policy notes: “Any social media engagement undertaken by Janssen UK will be imperfect due to the conflicting obligations a pharmaceutical company has to meet.”
The company also wants people to be allowed to comment and share their views “in as open a manner as possible”, and says it will accommodate this as far as it can “while working within the spirit of the ABPI Code of Practice” and its own business integrity guidelines.
• InPharm’s Making Digital Marketing Work For UK Pharma webcast was broadcast live on Wednesday 22 September and is now archived and available for viewing here
Dominic Tyer is web editor for Pharmafocus and InPharm.com and the author of the Digital Pharma blog. He can be contacted via email, Twitter or LinkedIn.
Related Content

Boehringer Ingelheim’s Spevigo gains additional approvals in US and China
Boehringer Ingelheim has announced that Spevigo (spesolimab-sbzo) has been approved by the US Food and …

GSK enters agreement for license for JNJ-3089 for development of bepirovirsen
GSK and Arrowhead Pharmaceuticals have announced that they have come to an agreement with Janssen …

Janssen and Sanofi enter agreement for potential vaccine programme
Janssen Pharmaceuticals, a Johnson & Johnson company, has announced a development and commercialisation agreement with …








