Digital Pharma: Bayer tweets breach ABPI Code of Practice
pharmafile | July 5, 2011 | News story | Medical Communications | Bayer, Code of Practice, Digital Pharma blog, PMCPA, Twitter, medical communications news, social media
Bayer’s use of Twitter has caused the first social media breach of the ABPI Code of Practice.
Code regulator the PMCPA ruled Bayer’s UK Twitter account did promote prescription-only medicines to the public when it sent two product-related tweets.
These concerned the company’s erectile dysfunction treatment Levitra and its multiple sclerosis spasticity drug Sativex, and were first reported by the Digital Pharma blog.
The company was found to have breached clause two of the Code (‘bringing discredit on, and reducing confidence in, the pharma industry’) and as a result may now be named in health trade journal advertisements.
The PMCPA also ruled breaches of clauses 22.1 (which bans advertising prescription-only medicines to the public) and 22.2 (which says information for the public must be factual and presented in a balanced way).
Finally the Panel also considered high standards had not been maintained and ruled a breach of clause 9.1.
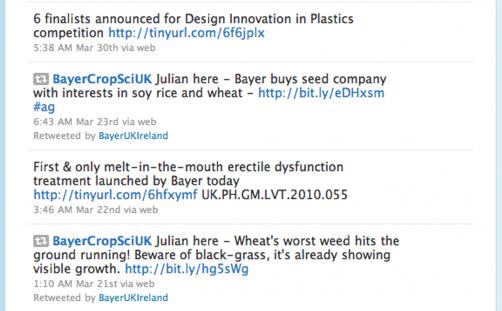
Bayer tweeted about Levitra in March this year, saying: “First & only melt-in-the-mouth erectile dysfunction treatment launched by Bayer today,” and included a link to a press release on its UK website.
An earlier tweet from last June went further and mentioned a brand name, indication and launch.
That tweet, which also include a link to a UK press release, read: “Sativex® launched in UK for the treatment of spasticity due to Multiple Sclerosis.”
Both tweets featured headlines from the press releases they linked to. The releases themselves had been signed-off internally for Code compliance, but the tweets had not.
The PMCPA also noted that the @BayerUKIreland Twitter account, which had about 500 followers at the time, was accessible by members of the public.
Promoting prescription-only medicines to the public is one of the Code of Practice’s cardinal sins and in its ruling the PMCPA said it considered Bayer to have made public announcements about the launch of a prescription-only medicine on Twitter.
“The Panel considered that each tweet promoted a prescription-only medicine to the public and would encourage members of the public to ask their health professionals to prescribe it,” the PMCPA report said.
The Code regulator was also concerned that material placed on Twitter had not been certified for use in that environment, noting that if part of a certified document is reproduced in a different format or directed to a different audience the new material should be certified separately.
The complaint was set in motion when PMCPA was contacted by “an anonymous healthcare reporter” (and not the author of this blog), following publication of a Digital Pharma post that questioned the tweets issued by Bayer.
The PMCPA ruling on the case did note that the use of social media, including Twitter, to provide information to the public was a legitimate activity for UK pharma, so long as the material complied with the Code of Practice, and clause 22 in particular.
This prohibits the advertising of prescription only medicines to the public (clause 22.1), while permitting information to be supplied directly or indirectly to the public, but only if such information is factual and presented in a balanced way (clause 22.2).
Bayer’s slip up coincided with the release of new PMCPA guidance on the use of digital media, which specifically addressed the restrictions on using Twitter to promote medicines. This all but ruled out such use, saying companies must ensure a healthcare professional-only audience and that recipients have agreed to receive the information.
Dominic Tyer is web editor for Pharmafocus and InPharm.com and the author of the Digital Pharma blog He can be contacted via email, Twitter or LinkedIn.
Related Content

Evotec and Bayer announce new kidney disease study
Evotec and Bayer have announced the initiation of a phase 2 clinical study in kidney …

Third application for Orion’s prostate cancer drug submitted by partner Bayer
Finnish pharmaceutical company Orion has announced that its partner Bayer is applying for a third application …

Bayer and Evotec to collaborate on precision cardiology
Bayer and Evotec have announced that they have updated the focus of their collaboration to …






