Digital Pharma: Bayer UK’s Twitter slip-up
pharmafile | April 26, 2011 | News story | Medical Communications | ABPI Code of Practice, Bayer, Digital Pharma blog, PMCPA, Twitter, medicial communications
There seems to be some confusion at Bayer UK over what communications can be sent over Twitter.
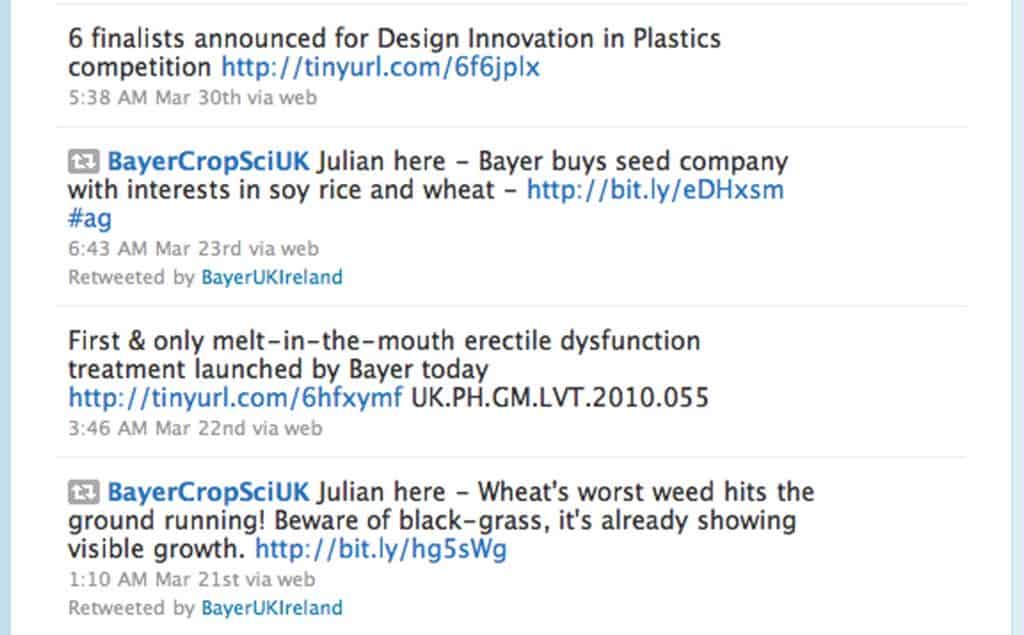
The company is using its @BayerUKIreland Twitter account to communicate updates from across its diverse business, including a tweet about a newly-launched version of its erectile dysfunction drug Levitra.
Last month Bayer tweeted: “First & only melt-in-the-mouth erectile dysfunction treatment launched by Bayer today http://tinyurl.com/6hfxymf.”
It links to a UK press release announcing the launch of a new formulation of Levitra that “dissolves on the tongue in seconds without the need for water, has a minty flavour and comes in a discreet pack”.
It’s not the first time Bayer has broadcast product information on Twitter. Last June the company tweeted: “Sativex® launched in UK for the treatment of spasticity due to Multiple Sclerosis http://tiny.cc/kiz2y.”
The two product-specific tweets, which the company deleted after being contacted by Pharmafocus, stick out like a sore thumb compared to those from the handful of other UK pharma Twitter accounts.
These not only have all their tweets signed off by medical and legal departments but also confine themselves to tweeting ‘disease awareness’ or healthcare news from the mainstream press.
When contacted about the tweet Bayer said it was “absolutely not” its intention to promote prescription medicines to the public.
Andrea Postles, PR & Media Relations Manager at Bayer UK, said: “We have sometimes ‘tweeted’ approved news releases where we know that the content, while of interest, is nevertheless non-promotional, and contains only factual and balanced information.
“All news releases are ABPI Code-approved before they are issued. Social media users only see our tweets if they have already elected to be ‘followers’.”
The @BayerUKIreland account has about 500 followers, some are clearly members of the public, but many show no information as to followers’ location or occupation. As an open account all its tweets are on public display and indexed by search engines like Google.
The company’s slip up coincides with the release of new PMCPA guidance on the use of digital media, but Bayer’s Andrea Postles said there were some limitations with this.
“Despite the recent publication of a discussion paper from the PMCPA, the position on use of social media and digital communication in relation to prescription medicines remains far from clear,” she said.
Nonetheless, the Code of Practice regulator did specifically address the restrictions on using Twitter to promote medicines in its new guidance, saying companies must ensure a healthcare professional-only audience and that recipients have agreed to receive the information.
“Given these restrictions and the character limit on Twitter, it is highly unlikely that the use of this medium to promote prescription-only medicines would meet the requirements of the Code,” the PMCPA concluded.
It did say pharma “should be able to use digital media”, but that it had to find ways to do so that avoided promoting prescription-only medicines to the public.
It also said social media can be used to provide information to the public – as long as the material complies with the Code.
Dominic Tyer is web editor for Pharmafocus and InPharm.com and the author of the Digital Pharma blog He can be contacted via email, Twitter or LinkedIn.
Related Content

Evotec and Bayer announce new kidney disease study
Evotec and Bayer have announced the initiation of a phase 2 clinical study in kidney …

Third application for Orion’s prostate cancer drug submitted by partner Bayer
Finnish pharmaceutical company Orion has announced that its partner Bayer is applying for a third application …

Bayer and Evotec to collaborate on precision cardiology
Bayer and Evotec have announced that they have updated the focus of their collaboration to …






