Digital Pharma: Roche tackles sidewiki
pharmafile | December 21, 2009 | Feature | Medical Communications | Roche, digi, digital pharma, google, sidewiki
Roche has become the second pharma company to tackle sidewiki on its corporate website, posting an entry that sets out its policy on the technology.
Google’s universal commenting tool, which uses a special sidebar to allow anyone to add a comment to any website, has caused some soul-searching in pharma.
Companies have struggled to come up with an appropriate response, leaving their websites peppered with user-generated entries that go unacknowledged.
Last month AstraZeneca became the first pharma company to add a sidewiki entry to its site, and this has recently been slightly updated, and more recently Sanofi-Aventis put an entry on its US product website for Lovenox.
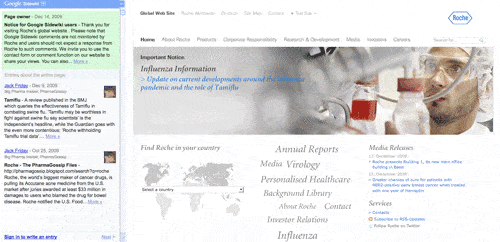
Roche’s solution is less obtrusive than AZ’s, which takes up the whole of the first page of sidewiki entries, making subsequent comments less visible by regulating them to later pages.
Instead the Swiss pharma company is using a smaller entry, that still allows users’ comments to appear on the first page, but makes its position clear – Google themselves have followed a similar path on their site.
Roche’s sidewiki thanks users for visiting the website, going on to say: “Please note that Google Sidewiki comments are not monitored by Roche and users should not expect a response from Roche to such comments. We invite you to use the contact form or comment function on our website to share your views.”
Behind the scenes the company has worked hard to agree a policy for the technology – needing as it does buy-in not just from IT (or more commonly these days, those charged with social media responsibility), but also legal and corporate communications.
Operating in ‘internet time’ is a challenging concept for any high-regulated industry, but it has become increasingly necessary as more applications look to follow Twitter’s lead and operate in ‘real-time’.
The next challenge might not be sidewiki – though it may well come from Google (2010 will after all see Google Wave rolled out more widely).
However, so far only three pharma companies have addressed sidewiki, despite the technology being released in September, so Google’s commenting tool provides a useful example of how the industry is currently tracking and responding to emerging online challenges.
Those pharma sidewiki texts in full:
Roche’s entry on Google sidewiki
Thank you for visiting Roche’s global website.
Please note that Google Sidewiki comments are not monitored by Roche and users should not expect a response from Roche to such comments.
We invite you to use the contact form or comment function on our website to share your views.
You can also follow us on Twitter or call the Roche switchboard at +41 61 688 1111.
If you are in the US, please visit rocheusa.com for information and contact.
For medical questions or to report any unexpected side effects related to one of our products, please contact your doctor first.
If needed, please contact the Roche office in your country.
Please note that Roche is not responsible for third party comments on Google Sidewiki and the opinions expressed do not represent the opinion of Roche.
We expressly disclaim any liability for all third party information and the use of it.
AstraZeneca’s entry on Google sidewiki
Google SideWiki comments posted on AstraZeneca.com are not monitored by AstraZeneca and users should not expect a response. You can follow AstraZeneca on Twitter or use the contact us form on this website.
AstraZeneca.com provides information about the AstraZeneca International business. If you are in the US you should visit AstraZeneca US. We also have national business sites which can be found under our list of AstraZeneca websites.
About AstraZeneca
AstraZeneca is one of the world’s largest biopharmaceutical companies. Researching, developing, manufacturing and marketing prescription medicines to treat gastrointestinal, cardiovascular, neuroscience, respiratory, oncology and infectious disease.
Available on AstraZeneca.com:
For investors, you can access our latest annual report, company presentations and read our shareholder FAQs.
Sanofi’s entry on Google sidewiki
Sidewiki is not a service provided or sponsored by sanofi-aventis U.S. Comments posted to this Sidewiki do not reflect the views and opinions of sanofi-aventis U.S. To contact sanofi-aventis U.S. directly, please visit https://contactus.sanofi-aventis.us or call 1-800-981-2491.
Dominic Tyer is web editor for Pharmafocus and InPharm.com and the author of the Digital Pharma blog. He can be contacted via email, Twitter or LinkedIn.
Related Content

Roche’s Alecensa approved by FDA as lung cancer treatment
Roche has announced that the US Food and Drug Administration (FDA) has approved Alecensa (alectinib) …

Genentech’s Columbi meets primary endpoint in phase 3 trial for lymphoma treatment
Genentech, part of the Roche Group, has announced that its phase 3 STARGLO trial has …

Lonza to acquire biologics site in Vacaville, US from Roche for $1.2bn
Lonza has announced that it has signed an agreement to acquire the Genentech large-scale biologics …








