Experts call for greater urgency in MS treatment
pharmafile | October 7, 2015 | News story | Research and Development | multiple sclerosis
A therapeutic strategy in multiple sclerosis (MS) that aims to maximise lifelong brain health (neurological reserve) needs to be widely accepted – and urgently adopted, says a new evidence-based report: Brain health: time matters in multiple sclerosis.
The report marks the start of a global initiative to improve outcomes for people with multiple sclerosis. In December 2014, lead author and neurologist Professor Gavin Giovannoni invited clinicians, researchers, specialist nurses, health economists and representatives from patient groups to form an international author group.
The multidisciplinary group undertook structured discussions between January and August 2015, and the report presents the evidence, consensus findings and recommendations that emerged from this process.
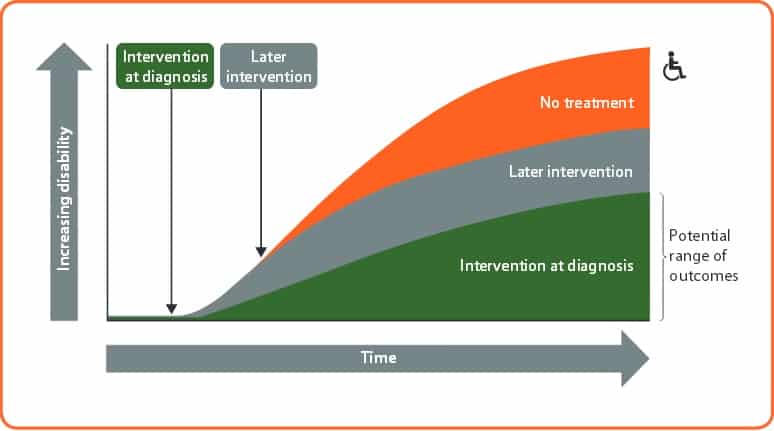
Their published strategy includes early intervention, a clear treatment target, regular monitoring and improved access to disease-modifying therapies (DMTs). A comprehensive approach to the management of MS, involving adopting a healthy lifestyle and preventing and treating comorbidities is also a vital component.
Evidence shows that DMTs are most effective early in the disease course. The Association of British Neurologists recommends starting treatment with a DMT “as early as possible in eligible patients” in its 2015 guidelines. But treatment initiation and switching are often delayed and may be subject to restrictions in licensing stipulations, prescribing guidelines and reimbursement policies.
“Time is critical to preserving brain volume, cognitive function and physical function, but there are delays at every stage in the treatment pathway,” says Professor Giovannoni. “The evidence shows we need to focus on early diagnosis and early intervention and offering effective DMTs that maintain cognitive function and maximise brain health. We need preventative strategies to delay progressive disease and stave off future disability.”
Multiple sclerosis (MS) is an incurable chronic disease in which the body’s own immune system destroys tissue in the brain and spinal cord.
Globally, the estimated number of people with MS has increased from 2.1 million in 2008 to 2.3 million in 2013. The majority of those diagnosed with MS are young, active people in their 20s and 30s, and about two-thirds of those affected are women.
The report, authored by an international multidisciplinary expert group, sets out a series of consensus recommendations aimed at achieving the best possible outcomes for people with MS.
The recommendations call for people with relapsing forms of MS to have access to the full range of available DMTs and to be involved in decisions about treatment initiation and timely switching when MRI or clinical evidence indicate disease activity.
The recommendations include:
- minimise delays in the diagnosis
- start treatment early, with a disease-modifying therapy and lifestyle measures
- encourage shared decision-making between people with MS and healthcare professionals
- manage MS holistically
- set an explicit treatment target
- monitor disease activity proactively
- act swiftly on suboptimal control of disease activity by considering switching DMT
- collect and record monitoring data to generate real-world evidence
- carry out economic evaluations from a societal perspective
- continue to investigate, develop and use cost-effective therapeutic strategies.
The recommendations are endorsed by a number of professional associations and advocacy groups, including the European Brain Council (EBC), the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Multiple Sclerosis Platform (EMSP).
Welcoming the report, Maggie Alexander, Chief Executive of EMSP, says: “This is a timely, challenging report that sets out important policy recommendations with the potential to have a significant impact on the lives of hundreds of thousands of people affected worldwide by multiple sclerosis.”
Marco Aurélio Lana-Peixoto, Chief Executive of the Brazilian Committee for Treatment and Research in Multiple Sclerosis (BCTRIMS), comments: “This report is an outstanding milestone to guide clinicians to approach MS appropriately, according to the most recent scientific evidence.”
Joel Levy
Related Content

Sanofi shares results from phase 2 trial for frexalimab in MS treatment
Sanofi has announced new phase 2 trial data for its CD40L monoclonal antibody, frexalimab, for …

Sanofi announce positive phase 2 data for MS drug frexalimab
French pharmaceutical and healthcare company Sanofi have announced positive trial data from its phase 2 …

Biogen announces collaboration on novel MS treatment
Biogen and InnoCare Pharma have announced a license and collaboration agreement for orelabrutinib, an oral …






