Pharma industry calls for EMA relocation by June in open EFPIA letter
pharmafile | April 25, 2017 | News story | Manufacturing and Production, Medical Communications, Research and Development, Sales and Marketing | EFPIA, EMA
Heads of research from 19 of the biggest players in the industry have called for a decision to be made on the new site of the EMA’s new headquarters by June – the date of their next scheduled meeting – in an open letter from the European Federation of Pharmaceutical Industries and Associations (EFPIA).
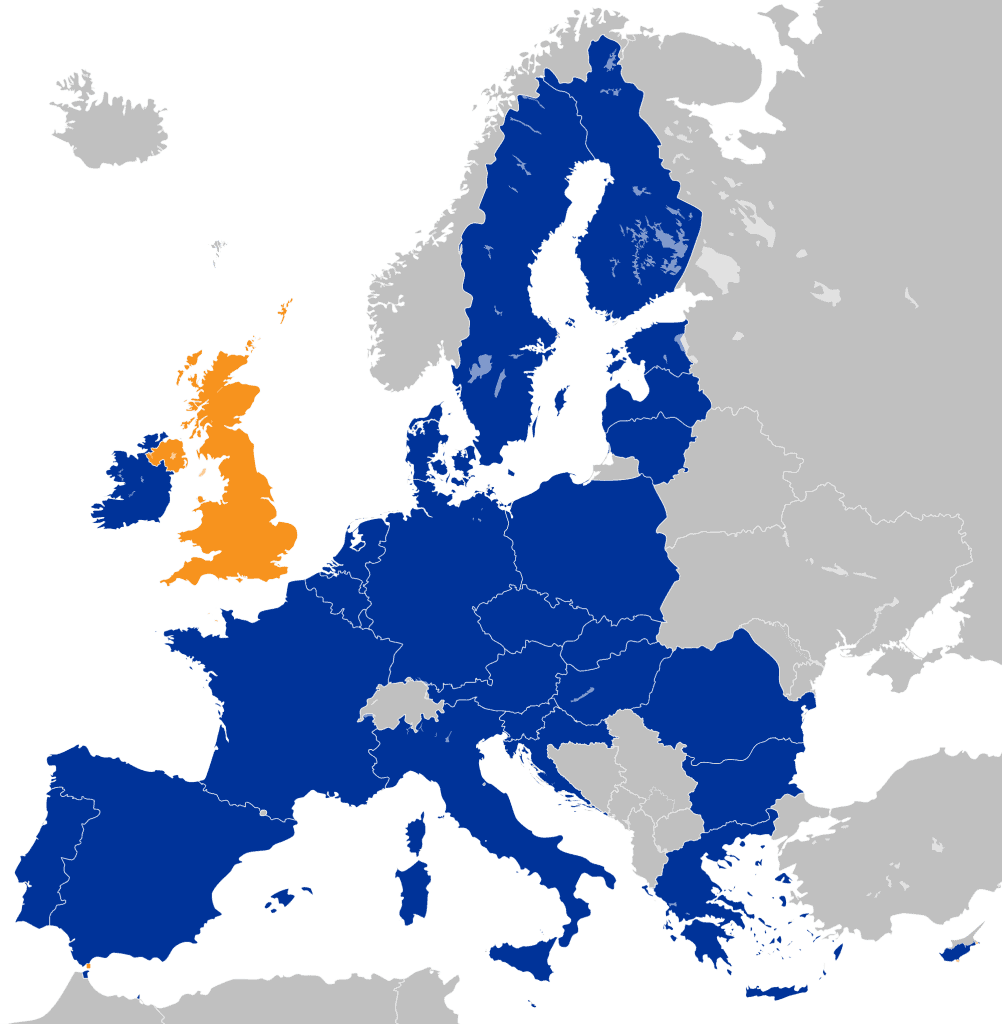
The EMA is currently headquartered in London. The letter highlights the danger posed by the UK’s impending exit from the EU, and the urgency of avoiding disruption to key operations that would occur if the EMA and its 900 staff cannot continue to function seamlessly at a new centralised site immediately.
“For over two decades, EU Member States have benefitted from and relied upon the critical work undertaken by the EMA in relation to the approval of new medicines, pharmacovigilance and safety monitoring activities,” the letter reads. “It is a stark and alarming reality that such fundamental activities would undoubtedly be impeded were the operations of the agency to be disrupted as a result of the United Kingdom’s exit from the EU. To put it concisely: in the event of obstruction or failure, Europe possesses no backup option.”
While 21 EU member states have vied for the right to host the new EMA, the letter was very particular about the requirements of a new headquarters, focusing centrally on “world-class connectivity”.
“This is a critical building block if the agency is to ensure that it is capable of managing and accommodating the 36,000 expert visits that it must facilitate currently on an annual basis, in addition to a plethora of regulatory exchanges with the global pharmaceutical industry. Equally important will be excellent transport links (international, regional and local transport), a building that is geared towards allowing the EMA to host the vast number of essential expert meetings it organises every year, and a location that is capable of furnishing a large number of hotel rooms that are prerequisite in order to host the wide range of experts who engage with the EU medicines agency to provide input into vital regulatory processes.”
The letter is signed by senior executives from Novartis, Bayer, Pfizer, Teva, Amgen, Ipsen, Eli Lilly, Boehringer Ingelheim, AstraZeneca, MSD, Takeda, Roche, Merck, Johnson & Johnson, Novo Nordisk, GSK and Sanofi, and makes clear the importance of a swift decision.
“Were a rapid resolution on the future location of the EMA not to materialise, or if the future seat of the European Medicines Agency were to fail in terms of establishing its minimum prerequisites, the quality of its work and the future of the European Medicines Regulatory Network would be placed in jeopardy. The extent of the severe and significant negative repercussions for public and animal health in Europe would be indeterminable.”
Matt Fellows
Related Content
EC approves Pfizer’s Prevenar 20 to protect paediatric patients against pneumococcal disease
Pfizer has announced that the European Commission (EC) has granted marketing authorisation for Prevenar 20, …

EMA validates two applications for datopotamab deruxtecan for cancer treatments
AstraZeneca has announced that the European Medicines Agency (EMA) has validated to marketing authorisation applications …

AstraZeneca’s Voydeya recommended for approval in EU by CHMP
AstraZeneca has announced that Voydeya (danicopan) has been recommended for marketing authorisation in the European …







