Pfizer’s self-injectable contraceptive becomes UK’s first
pharmafile | September 30, 2015 | News story | Medical Communications, Research and Development, Sales and Marketing | Pfizer, Syayana Press
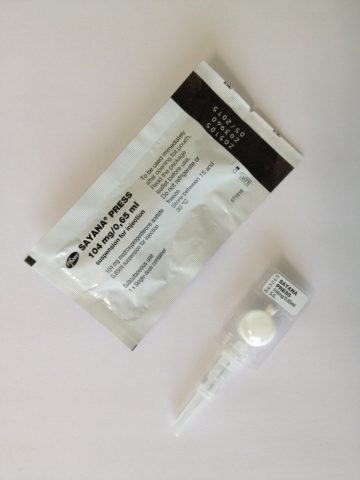
Pfizer’s injectable contraceptive, Sayana Press (medroxyprogesterone acetate), is now available to women in the UK for administration by self-injection following approval from the UK Medicines and Healthcare Products Regulatory Agency (MHRA) of an update to the Sayana Press label.
The label now gives the option for self-injection by women when considered appropriate by a healthcare professional.
Sayana Press is the first injectable contraceptive in the UK available for administration via self-injection. This new method of administration is also approved or pending local approval in additional EU markets including Austria, Belgium, Hungary, Ireland, Netherlands.
Sayana Press combines a long-acting, reversible, contraceptive with an all-in-one prefilled, single-use, non-reusable injection system, eliminating the need to prepare a needle and syringe.
Approved for use by the MHRA in 2011, the contraceptive is indicated for the prevention of pregnancy. Each subcutaneous injection prevents ovulation and provides contraception for around 13 weeks.
Injectable contraceptives are a widely-used family planning method and are seen as a discreet method that eliminates the need for a daily pill regimen.
Dr Salomon Azoulay, senior vice president and chief medical officer, Pfizer says: “With this revised label, following consent from a healthcare professional and with proper training, UK women will now have the opportunity to administer Sayana Press outside of a clinical setting.
“This is an exciting milestone for women in the United Kingdom, and, potentially, in countries around the world, who might prefer this method of contraception and mode of administration.”
Pfizer said it would continue to help bring this updated label to more countries worldwide, with an initial focus on those in the developing world, as Sayana Press is not yet approved for self-injection outside of the EU.
Yasmita Kumar
Related Content
NICE recommends Pfizer’s new once-weekly treatment for haemophilia B on NHS
Walton Oaks, 21st May 2025 – Pfizer Ltd announced today that the National Institute for Health and Care …

Pfizer releases results for severe RSV-associated LRTD treatment study
US-based Pfizer have announced results from its substudy B of the ongoing phase 3 clinical …
New Real-World Data Published in Journal of Cardiac Failure on Effectiveness
Patients treated with tafamidis were associated with greater rates of survival compared with patients untreated …






