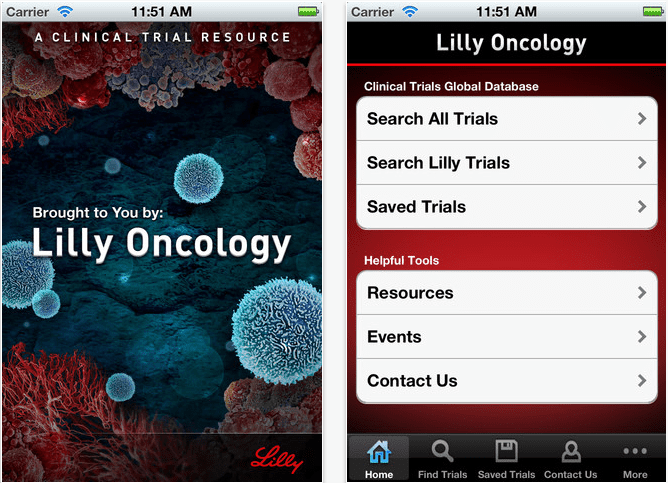
Lilly app aims to boost trial recruitment
pharmafile | June 8, 2012 | News story | Medical Communications | GSK, clinical trials, lilly, oncology
Lilly has launched a new searchable clinical trial mobile app for oncologists.
The app is available for the iPad and iPhone as well as BlackBerry and Android platforms, and allows healthcare professionals to search oncology trials that are enrolling new patients.
The firm said that doctors could search all oncology trials via its mobile software, not just ones from Lilly. The app allows doctors to contact Lilly Oncology for additional details on its trials, as well as a third-party contact for the non-Lilly clinical trials.
But doctors may be wary that an app designed by a pharma firm will encompass all trials, and may decide to use the ‘Clinical Trials’ app, which allows the user to search all trials available on the clinicatrials.gov site.
It is available on iTunes for £2.49 – the Lilly app is free, but having its name attached to the app could meet with low uptake. Lilly is not the first pharma firm to create an app for this purpose, with GlaxoSmithKline creating a similar app last year.
The reason behind the app is to increase enrollment in clinical cancer trials by allowing doctors to search for oncology trials on their smartphone or tablet. By having a new interactive medium, firms such as Lilly and GSK also hope to raise awareness about their own studies.
Anne White, senior director of portfolio management with Lilly Oncology, said: “Lilly Oncology created the ‘Clinical Trial Resource’ mobile app to offer cancer care professionals an easy way to search for and identify details about all global oncology clinical trials.”
Directions for downloading the clinical trial app are available on a new website, LillyOncologyPipeline.com
On the site healthcare professionals can search for information about Lilly Oncology’s pipeline by drug discovery platform, cancer type, clinical trial Phase and molecular target.
Specific information about each medicine includes illustrations of the target pathway and also includes videos of the compound’s method of action.
Ben Adams is the reporter for Pharmafocus and InPharm.com and DigiBlog site author. He can be contacted via: email or Twitter.
Related Content

BMS’ Opdivo/Yervoy combination accepted by Scottish Medicines Consortium for colorectal cancer
Bristol Myers Squibb (BMS) has announced that its Opdivo (nivolumab) has been accepted, in combination …

Astellas Pharma’s Vyloy accepted by Scottish Medicines Consortium for gastric cancer
Astellas Pharma, a pharmaceutical company creating medicines to address unmet medical needs, has announced that …

GSK’s Exdensur receives MHRA approval for asthma and rhinosinusitis
GSK’s Exdensur (depemokimab), a twice-yearly biological medicine, has received approval from the UK Medicines and …





