Latest news from Datamonitor
pharmafile | February 6, 2008 | News story | Sales and Marketing | Coronado Biosciences, rnai
Coronado Biosciences’ cancer treatment effective in animal models
Coronado Biosciences has reported that the Bcl-2 inhibitor, apogossypol, effectively killed a variety of cancer cells in vitro while demonstrating less toxicity than its parent compound gossypol in vivo in mice.
Published online in Blood by the Burnham Institute’s president and CEO, John Reed and collaborators, the paper summarized study findings demonstrating that mice treated with apogossypol experienced less hepatotoxicity and gastrointestinal toxicity than those treated with gossypol. The results support Coronado Biosciences’ continued development of its lead candidate, CNDO103, as a cancer treatment.
Dr Reed said: “This study provides strong evidence that apogossypol is better tolerated by the animals without a compromise in potency in vitro. We hope these preclinical results translate to humans. If they do, apogossypol can play a role in the treatment of many types of cancers.”
Coronado Biosciences plans to perform IND-enabling preclinical studies for CNDO103 in the first half of the year and if the toxicity profile in animals is favorable, the company plans to file an IND in late 2008.
Related Research
Pipeline Insight: Molecular Targeted Cancer Therapies Can anything else revolutionize the market?
The Cancer Market Outlook to 2012
Alnylam receives UK patent for RNAi therapeutics
Alnylam Pharmaceuticals, a RNAi therapeutics company, has announced that the UK Patent Office has granted a patent for the Woppman patent series.
The newly granted patent includes 32 claims broadly covering compositions and methods, including pharmaceutical compositions, for small interfering RNAs (siRNAs), the molecules that mediate RNAi. The claims cover siRNA molecules of any length that contain ‘overhang’ and ‘blunt end’ design features, including siRNAs containing chemical modifications and certain novel motifs.
Barry Greene, president and COO of Alnylam, said: “With this progress, we are extending the scope of our comprehensive ‘first-mover’ consolidation of early filed RNAi fundamental patents and patent applications.”
Related Research
Outlook for RNAi, 2007: siRNA and miRNA in biology, diagnostics and therapeutics
Bausch & Lomb and CrystalGenomics sign R&D agreement
Bausch & Lomb and CrystalGenomics have signed a joint research and development agreement to study potential new treatments for inflammatory ophthalmic diseases.
CrystalGenomics will provide new pharmaceutical candidate compounds while Bausch & Lomb will develop product formulations, conduct pre-clinical and clinical studies, and bring the products to market.
Praveen Tyle, chief scientific officer of Bausch & Lomb, said: “We believe this collaboration has the potential to extend our leadership in anti-inflammatory ophthalmic pharmaceuticals, especially with CrystalGenomics’s unique 3D structure-based optimization technology for compound discovery.”
Related Research
Outlook for RNAi, 2007: siRNA and miRNA in biology, diagnostics and therapeutics
Translational Medicine in Biopharmaceutical R&D
Nanotechnology: Revolutionizing R&D to develop smarter therapeutics and diagnostics
BioLineRx’s pain drug found effective in preclinical trials
BioLineRx has reported positive results from pre-clinical trials, which demonstrated that BL-1021 was safe and effective in pre-clinical testing for the treatment of neuropathic pain.
In pre-clinical trials, BL-1021 showed a significant reduction in symptoms of neuropathic pain with reduced side effects. The molecule was given orally and in all cases was found to be superior to available treatments due to its increased efficacy and minimal side effects. Furthermore, the experiments demonstrated that BL-1021 demonstrated potent analgesia drug without noticeable side effects of sedation and cardiac arrhythmias, which often plague other anti pain compounds.
BL-1021, an orally available small molecule for the treatment of neuropathic pain is being developed by BioLineRx under a worldwide exclusive license from Ramot at Tel Aviv University and Bar-Ilan Research and Development Company, the technology transfer arms of Tel Aviv University and Bar-Ilan University.
Morris Laster, CEO of BioLineRx, said: “We believe that BL-1021 has the potential to be a safe and effective drug for neuropathic pain, and we look forward to the initiation of the clinical trial program for this compound in 2008.”
Related Research
Pipeline Insight: Neuropathic Pain – Emerging drugs fail to capitalize in lucrative market
Patient Recruitment and Retention in Clinical Trials: Emerging strategies in Europe, the US and Asia
Medivation to initiate confirmatory Phase III Alzheimer’s trial
Medivation has announced that based on its end-of-Phase II meeting with the FDA, the company plans to begin a pivotal confirmatory Phase III trial of Dimebon for mild-to-moderate Alzheimer’s disease in the second quarter of 2008.
The FDA informed Medivation that the company’s previously completed trial conducted in Russia can be used as one of the two pivotal studies required to support the approval of Dimebon to treat mild-to-moderate Alzheimer’s disease, as long as a significant proportion of the sites in the confirmatory Phase III trial are located in the US.
The Phase III clinical trial will enroll approximately 525 patients with mild-to-moderate Alzheimer’s disease at sites in the US, Europe and South America. Patients will be randomized to one of three treatment groups: Dimebon 20mg three times per day (TID), Dimebon 5mg TID, and placebo. Patients will be treated for six months and may not be taking any other Alzheimer’s disease drugs.
The primary endpoints are the Alzheimer’s disease assessment scale – cognitive subscale (ADAS-cog) and the Clinician’s interview-based impression of change plus caregiver interview (CIBIC-plus). Medivation expects to complete the pivotal confirmatory Phase III trial and apply for marketing approval in 2010.
David Hung, president and CEO of Medivation, said: “We are now a Phase III company with clear regulatory guidance on the pivotal trials required to seek marketing approval for Dimebon in the US.”
Related Research
Related Content

Alnylam and Roche partner for RNAi therapeutic for treatment of hypertension
Alnylam Pharmaceuticals has announced that it has entered a strategic agreement with Roche for the …
Alnylam cuts workforce by a third
Alnylam will cut 33% of its workforce this year as it looks to focus on …
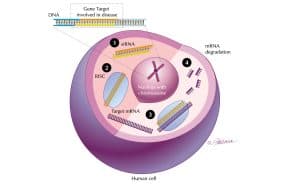
RNA interference – still the great white hope?
The emerging field of RNA interference – also known as ‘gene silencing’ – is one …






