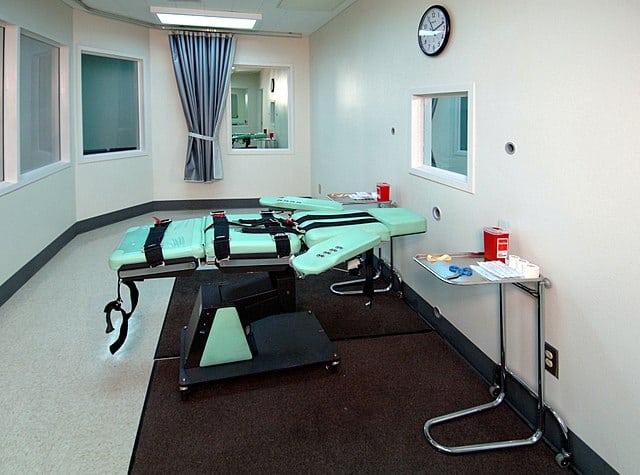
Janssen opposes use of its drug in state execution
pharmafile | August 23, 2017 | News story | Medical Communications, Research and Development, Sales and Marketing | Janssen, etomidate, life sciences, pharma, pharmaceutical
Janssen has spoken out against plans to use an anaesthetic drug it invented over 50 years ago to execute prisoners, adding its voice to many others in the pharmaceutical industry who have taken similar stances in recent years.
Etomidate is planned to be used as part of a lethal three-drug cocktail to kill Mark Asay, a prisoner found guilty of two murders committed in 1987 – the first use of the drug in an execution. While Janssen has never sold etomidate in the US, and multiple generic versions are available as the drug is off-patent, the company made a point to oppose its use in applications of capital punishment.
“Janssen discovers and develops medical innovations to save and enhance lives. We do not support the use of our medicines for indications that have not been approved by regulatory authorities,” wrote Greg Panico, a spokesman for Janssen, in an email. “We do not condone the use of our medicines in lethal injections for capital punishment.”
The Florida Department of Corrections would not disclose which company is supplying the drug for the execution, which is due to take place on Thursday 24 August at 6pm local time.
“The Florida Department of Corrections follows the law and carries out the sentence of the court, as laid out in Florida Statute. This is the Department’s most solemn duty and the foremost objective with the lethal injection procedure is a humane and dignified process,” explained Michelle Glady, spokesman for the Florida Department of Corrections, said in an email.
Moves like Janssen’s to decry such uses of their drugs have succeeded in creating shortages of drugs used for lethal injections, forcing states to explore other methods and routes of supply. In the past, Pfizer implemented a strict distribution policy to ensure its version of midazolam could not be used in this same way.
“The American pharmaceutical industry is united in its view that it doesn’t want its medicines misused for nonmedical purposes, and killing prisoners has never been an approved medical purpose,” said Robert Dunham, Executive Director of the Death Penalty Information Center, an impartial organisation which criticises the application of capital punishment. “Because of secrecy laws, secrecy practices, the public simply doesn’t know why states are doing what they’re doing.”
Matt Fellows
Related Content

Lilly opens fourth US Gateway Labs site
Eli Lilly has opened its newest Lilly Gateway Labs (LGL) site in San Diego, California, …

LGC Group opens $100M Organic Chemistry Synthesis Centre of Excellence
LGC Group, a life sciences company, has opened its new Organic Chemistry Synthesis Centre of …

Crown Bioscience opens new US model development centre
Crown Bioscience, a US global contract research organisation (CRO) that is part of JSR Life …






