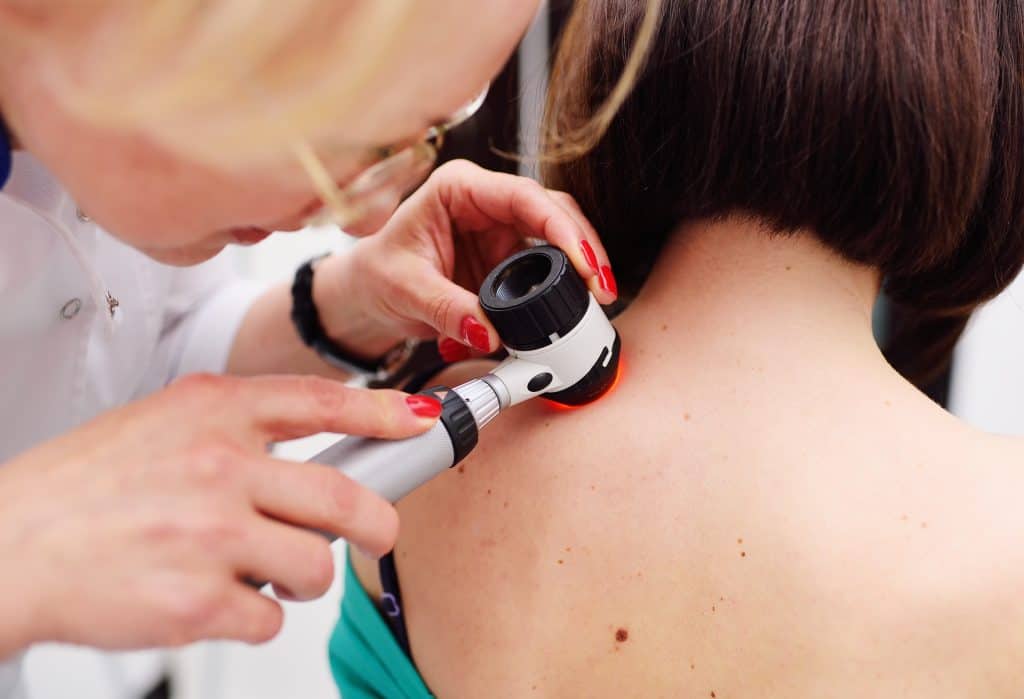
Iovance’s Amtagvi gains FDA accelerated approval for melanoma treatment
Betsy Goodfellow | February 19, 2024 | News story | Medical Communications | Iovance, Oncology, accelerated approval, melanoma
Iovance Biotherapeutics has announced that the US Food and Drug Administration (FDA) has approved Amtagvi (lifileucel) suspension for intravenous infusion for the treatment of adult patients with unresectable or metastatic melanoma, previously treated with a PD-1 blocking antibody and, if BRAF V600 mutation positive, a BRAF inhibitor with or without a MEK inhibitor.
The drug was approved under an accelerated approval based on its overall response rate (ORR) and duration of response. The company is currently conducting the TILVANCE-301 phase 3 trial to confirm the drug’s clinical benefit.
The drug is the first and only one-dose, individualised T-cell therapy to have gained approval by the FDA for a solid tumour cancer.
Frederick Vogt PhD JD, interim chief executive officer and president of Iovance, commented: “The accelerated approval of Amtagvi is the first step in realising Iovance’s ambition to usher in the next generation of cell therapy by bringing this breakthrough to patients with advanced solid tumours. Given the significant unmet needs in the advanced melanoma community, we are proud to offer a personalised, one-time therapeutic option for these patients. We are continuing our development efforts to address additional unmet medical needs in patients with solid tumour cancers, making our novel cell therapies available to more patients with melanoma and other types of cancers.”
Samantha R Guild JD, president, AIM at Melanoma Foundation, added: “The approval of Amtagvi offers hope to those with advanced melanoma who have progressed following initial standard of care therapies, as the current treatment options are not effective for many patients. This one-time cell therapy represents a promising innovation for the melanoma community, and we are excited by its potential to transform care for patients who are in dire need of additional therapeutic options.”
Betsy Goodfellow
Related Content

Five facts about UV exposure and sun safety
1We can acclimatise to UV exposure. For example, we’re more likely to burn if there’s …
Adaptimmune receives US FDA Accelerated Approval for engineered cell therapy for a solid tumour
Adaptimmune Therapeutics has announced today that the US Food and Drug Administration (FDA) has given …
INDEPENDENT PARSORTIX STUDY HIGHLIGHTS BENEFITS OF COMBINED DNA ANALYSIS IN MELANOMA
Multi-analyte approach has potential to guide personalised cancer care CTC mutation profiles associated with more …






