
FDA approves supplemental drug application for Pfizer’s EURCRISA in children with eczema
pharmafile | March 25, 2020 | News story | Research and Development, Sales and Marketing | eczema, eczema cream, eczema ointment
Pfizer announced that the FDA has approved its supplemental New Drug Application for EUCRISA ointment in treating 3 month year old children with mild-to-moderate atopic dermatitis, also known as eczema.
The ointment had previously been approved for adults and children who were over 2 years old. This new approval comes after a Phase 4 clinical trial which investigated how the treatment affected infants from 3 to 24 months old. The study lasted four weeks, and included 137 patients. The ointment was effective and well tolerated and there were no new safety concerns identified.
It is the first and only 100% steroid free ointment now available for young children. Speaking about the approval, Richard Blackburn, Global President, Inflammation & Immunology at Pfizer, said: “Despite atopic dermatitis often manifesting during infancy, there are few approved treatment options for this population available today. We are committed to making a meaningful difference to patients’ lives, and with this indication extension, we look forward to now helping many of the youngest children suffering with eczema.”
Eczema is a chronic skin disease that affects nearly 18 million people and 11% of all children in the US. 45% of eczema cases begins within the first six months of life and 60% of cases being in the first year.
Conor Kavanagh
Related Content
MHRA Grants Marketing Authorisation for LEO Pharma’s Anzupgo® ▼(delgocitinib) cream in Great Britain
The Medicines and Healthcare Products Regulatory Agency (MHRA) has granted marketing authorisation in Great Britain …

UC San Diego School of Medicine researchers treat atopic dermatitis with bacteriotherapy
Researchers at the University of California San Diego School of Medicine have identified a universal …
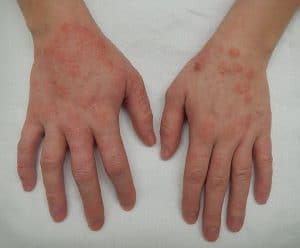
LEO Pharma’s delgocitinib hits main goals in moderate-to-severe chronic hand eczema
LEO Pharma has revealed new Phase 2b at the European Academy of Dermatology and Venereology …






