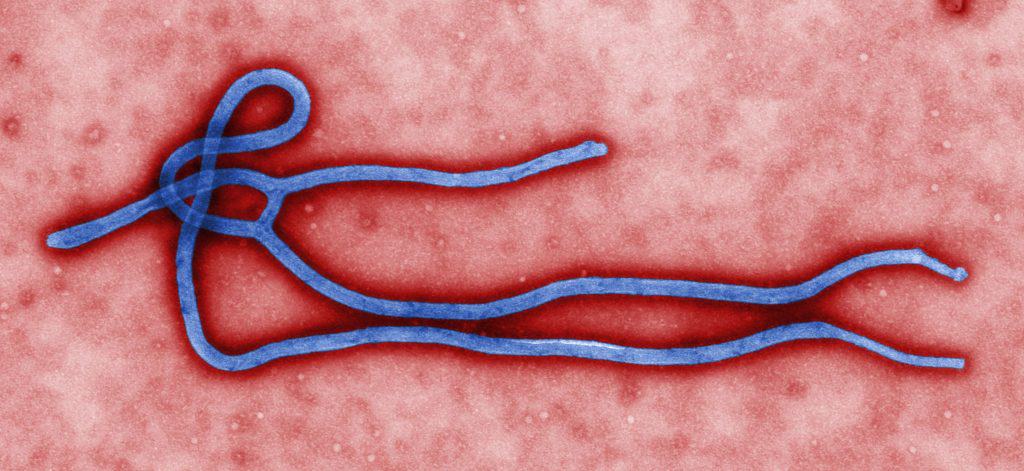
FDA approves Merck & Co’s Ebola vaccine
pharmafile | December 20, 2019 | News story | Medical Communications | Ebola, Ebola Drug, Ebola West Africa, Ebola vaccine, Merck
The FDA has approved Merck & Co’s Ebola vaccine, Ervebo. This marks the first time they have approved a vaccine against the virus.
It follows the company getting approval from the European Commission to market Ervebo back in November. The vaccine is administered through a single-dose to prevent EVD caused by Ebola in patients aged 18 or older.
Ervebo has already been used by the World Health Organisation (WHO) and the Democratic Republic of the Congo (DRC) to help reduce Ebola outbreaks during the West African epidemic from 2013-2016.
There have been more than 3,300 cases of Ebola in the DRC, which has led to 2,200 deaths. This caused the WHO, back in July, to classify the DRC outbreak as a Public Health Emergency of International Concern. It has been the worst outbreak of the disease since the 2013-2016 epidemics in West Africa.
Ebola was first discovered in the region where the DRC now covers in 1976. It is a virus that spreads through the body damaging the immune system and organs. It causes fever, body aches and sometimes internal and external bleeding.
In recent times, the most deadly Ebola outbreak came from Guinea in late 2013, and spread across West Africa. Over 11,000 people died from this particular epidemic.
Conor Kavanagh
Related Content
Merck to acquire Curon Biopharmaceutical’s B-Cell Depletion Therapy
Merck have announced that they have entered into an agreement with private biotechnology company Curon …
Merck and Daiichi Sankyo expand development and commericalisation agreement to include MK-6070
Daiichi Sankyo and Merck (known as MSD outside of the US and Canada) have announced …

CHMP gives positive opinion for Merck’s KEYTRUDA for unresectable or metastatic urothelial carcinoma
Merck (known as MSD outside of the US and Canada) has announced that its anti-PD-1 …






