FDA approves GBT’s Oxbryta to treat sickle cell disease
pharmafile | November 26, 2019 | News story | Manufacturing and Production | FDA, FDA Trump, Oxbryta, Sickle cell, sickle cell disease
The FDA announced yesterday that it has approved Global Blood Therapeutics’ marketing application for its haemoglobin polymerisation inhibitor Oxbryta, which is used to treat sickle cell disease.
In a statement, Acting FDA Commissioner Brett P Giroir said that: ‘‘Today’s approval provides additional hope to the 100,000 people in the US, and more than 20 million globally, who live with this debilitating blood disorder.’’ He added that the FDA remains ‘‘committed to raising the profile of this disease as a public health priority and to approving new therapies that are proven to be safe and effective.’’
Dr Richard Pazdur, Director of the FDA’s Oncology Center of Excellence and Acting Director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, said that Oxbryta ‘‘provides a new treatment option for patients with this serious and life-threatening condition.’’
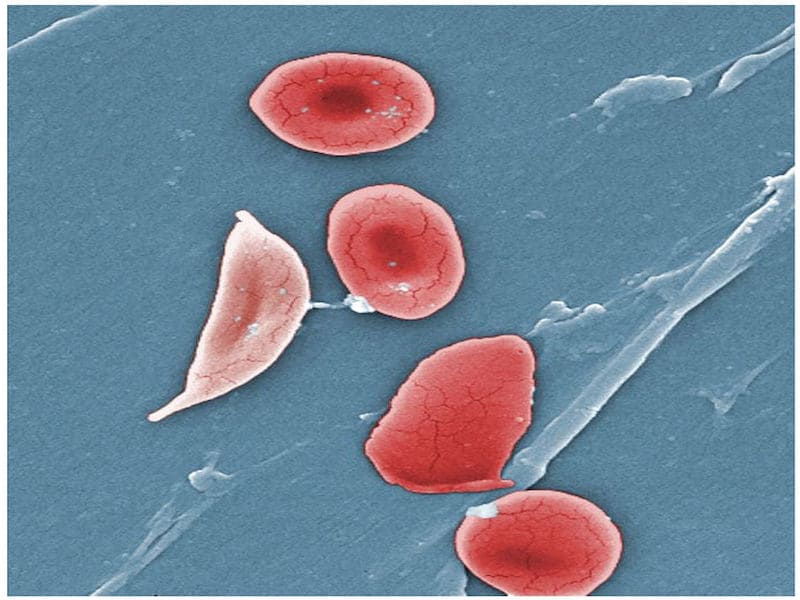
Sickle cell disease affects millions across the world, but mainly people from an African or Afro-Caribbean background. The disease is caused by a mutation in the gene for haemoglobin, the oxygen carrying molecule found in red blood cells. This mutation produces ‘sickle’ shaped blood cells that do not live as long as healthy blood cells and can block blood vessels. The lack of haemoglobin limits oxygen delivery to the body’s tissues leading to severe pain and organ damage.
Oxbryta was found to reduce the frequency of pain crises compared to its placebo in the clinical trial conducted by GBT. They believe boosting haemoglobin with their drug will lower incidents of stroke and preserve organ function in sufferers of sickle cell disease. However critics back in June raised concerns that patients may not benefit that much from the drug treatment, following the report of the trial in the New England Journal of Medicine.
Conor Kavanagh
Related Content

MRM Health’s ulcerative colitis treatment receives FDA Investigational New Drug clearance
Microbial Resource Management (MRM) Health has announced that its lead programme, MH002, has received Investigational …

Complement Therapeutics’ geographic atrophy treatment receives FDA Fast Track designation
Complement Therapeutics has announced that CTx001, its gene therapy treatment for geographic atrophy (GA) secondary …

Johnson & Johnson submits robotic surgical system for De Novo classification
Johnson & Johnson has announced the submission of its Ottava Robotic Surgical System for De …






