Cancer Fund improving treatment access
pharmafile | September 19, 2011 | News story | Sales and Marketing | Cancer Drugs Fund, postcode prescribing
The Cancer Drugs Fund is dramatically improving access to new cancer medicines for patients in England – but its success is creating fears of postcode prescribing across the UK.
The Cancer Drugs Fund (CDF) was widely criticised after the first interim fund was established nearly a year ago, with complaints made about the difficulty of administering the fund. There were also early signs of an under spend in many CDF regional funds, but it now looks that these problems are being addressed.
Crucially, the CDF allows patients to access cancer drugs before NICE has issued its final appraisal, as well as funding drugs rejected by NICE.
Pre-NICE funding is helping to end the long running problem of ‘NICE blight’, where regional funding is delayed until NICE issues its decision.
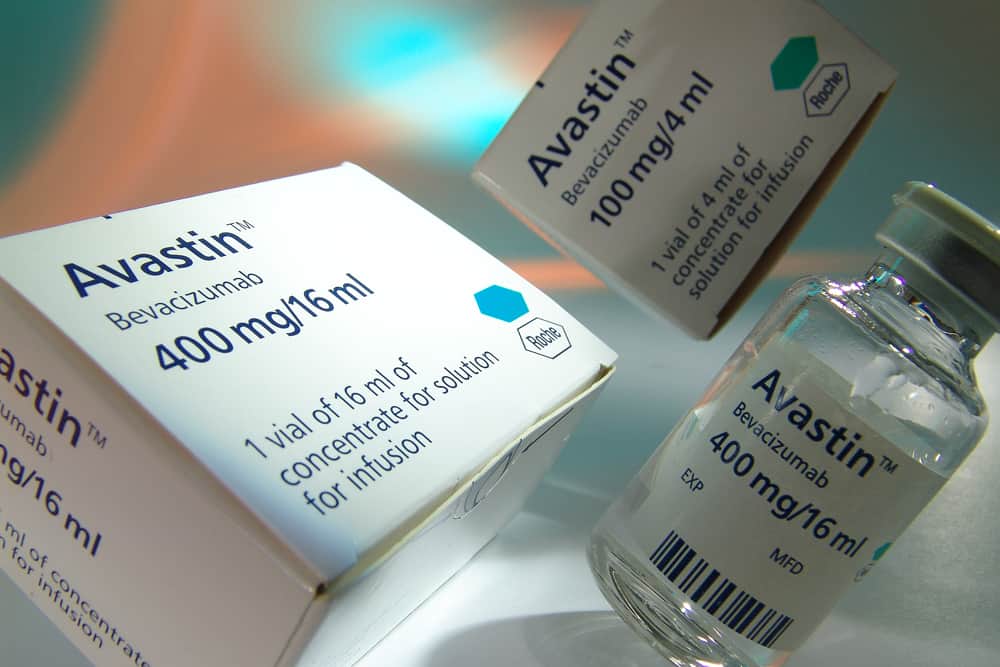
The £600 million fund is now giving more patients access to new cancer drugs more quickly, with Roche’s Avastin proving the most popular drug under the fund and between October 2010 and March 2011 1,040 funding requests for the drug were approved in England.
Nevertheless the ABPI has played down the encouraging signs from the CDF so far. It told Pharmafocus the CDF is a “real opportunity” to improve access to innovative medicines, but did not say it had yet achieved that goal.
In contrast, the Rarer Cancers Foundation says England’s CDF is a “resounding success” and having an increasing impact as doctors and patients become more aware of it.
But the success of England’s new fund is such that it is opening up a divide between England and Scotland and Wales in terms of access to cancer drugs.
A new report by the Rarer Cancers Foundation entitled Nations Divided? has found that Scotland and Wales are lagging behind England in access because of the CDF.
Overall, NHS cancer patients in England are more than three times more likely than those in Scotland to gain access to new drugs which are not routinely available through other means.
Just a year ago this situation was the reverse, but the extra funding from the CDF has rapidly changed the picture.
The charity is calling for a similar fund to be established in Scotland and Wales, adding that a “devastating divide” had opened up between the three nations on accessing these treatments.
Bristol-Myers Squibb’s skin cancer drug Yervoy (ipilimumab) is the latest new cancer treatment to be launched in the UK and it had already been made available to some patients within a week of its launch in August.
Two regions in England have already confirmed funding for Yervoy, a full six months before NICE will issue its guidance on the drug.
Postcode prescribing
But the success of the regionally-controlled funding brings with it the spectre of postcode prescribing. For example, only the North West and West Midlands regions in England have confirmed that Yervoy will be funded by the CDF, but the remaining eight have not.
This means that patients will have to wait longer in these regions to access the drug – or not have access to it at all.
The ABPI says such regional variations need to be ironed out. Moreover, the ABPI says it is concerned that the regional funds may not be spent in full, as was the case with the interim CDF.
The Rarer Cancers Foundation is also concerned about postcode prescribing, and points out that it inevitably arises because of the regional decision-making process.
“Health bodies should be looking to each other” to establish some sort of centralised system between the disparate health bodies, a spokeswoman said.
“Having separate priority lists and telling patients in one area that they can’t have a drug because ‘it’s not on the list’ whilst others are allowing these drugs will only disadvantage patients,” she added.
The £600 million fund is due to be discontinued when a value-based pricing (VBP) system is introduced in 2014. The ABPI says it wants to see “an integrated pricing and reimbursement scheme” in three years time, which might, in theory, allow access to other high cost medicines currently barred by NICE.
But a great deal of uncertainty remains around VBP, and some in the industry fear it could be no improvement on the current situation in terms of access to new medicines.
Ben Adams
Related Content

Pfizer’s Ibrance combo authorised via the Cancer Drugs Fund for advanced breast cancer
NICE, the drug watchdog for England and Wales, has given its approval for Pfizer’s Ibrance …

Pfizer’s Ibrance approved by NICE, to be added to the Cancer Drugs Fund
Today it was announced that the National Institute for Health and Care Excellence (NICE) has …
NICE rejects Keytruda for routine use in NHS bladder cancer treatment
The National Institute for Health and Care Excellence (NICE) has given an initial ‘no’ for …






