Taking on IFP, COPD and severe asthma: ERS 2015 roundup
pharmafile | October 9, 2015 | Feature | Medical Communications, Research and Development |
Leading pharmaceutical companies, physicians, healthcare professionals, scientists, respiratory experts, and of course the media, including Pharmafocus, gathered in Amsterdam at the end of September, for the annual European Respiratory Society Congress.
The scientific and educational programme delivered a wealth of new material from all sections of respiratory health and disease, with new clinical trial data for treatments for Idiopathic Pulmonary Fibrosis, COPD and asthma proving particular highlights.
Asthma
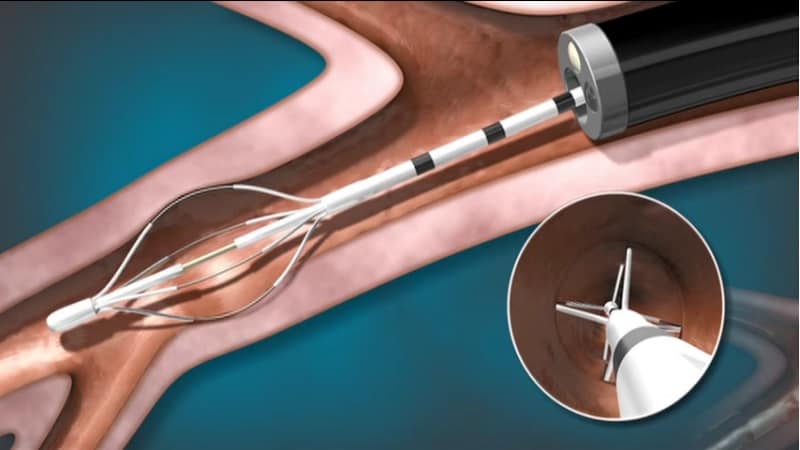
Bronchial Thermoplasty offers severe asthma sufferers hope without steroid treatment
Boston Scientific revealed the results of a new Europe-wide survey, revealing for the first time the impact of severe asthma on sufferers’ lives.
Boston Scientific presented results showing the condition affects a quarter of sufferers on a daily basis, and a huge 71% at least once a week.
Speaking at the Congress, a UK-based severe asthma sufferer, Rhea Yarworth, revealed the distress caused by living with the condition and how it affected her daily activities. Two years ago, she was given a Bronchial Thermoplasty treatment over three sessions.
Bronchial Thermoplasty has been FDA-approved since 2010, but as a consequence, safety and efficacy data is limited to five years. However, the procedure, which involves the removal of airway smooth muscle has proven effective for this duration in those patients that have received it.
Boston Scientific demonstrated its Alair system, by which it delivers the procedures, at the Congress.
Given the opportunity to have this procedure, Yarworth leapt at the chance. She found the second of three treatments difficult due to being conscious throughout, and was in some discomfort, which is believed to be rare. However, the final procedure was performed under general anaesthetic, and Rhia told Pharmafocus the outcome was certainly worth it, making her significantly more physically active and confident.
The new option of Bronchial Thermoplasty marks shift in mindset away from the one-size-fits-all approach currently seen in asthma treatment, and towards a more personalised, bespoke approach. This was very much a theme of the asthma coverage at the Congress.
Roche says the time is right for a personalised approach to severe asthma treatment
Asthma is an extremely common disease, affecting close to 334 million people worldwide. Although the sufferers and their symptoms are diverse, the condition is usually treated with a stepwise approach with medications designed to relieve symptoms, but which fail to tackle the underlying causes.
This stepwise approach begins with reliever therapies, before adding preventers and additional treatments proportionate to the increased severity of the asthma. Despite the large number of therapies often used, symptoms and vulnerabilities to attacks often remain.
At a briefing at ERS Congress 2015, Roche detailed its personalised healthcare strategy for bespoke therapies appropriate to the inherited or acquired risk factors displayed by different patient subgroups.
Scientists at the company are attempting to address the unmet patient need by the mapping the biological pathways involved in the inflammatory process, and resultantly have identified new therapeutic targets that may moderate the inflammatory response in a certain subset of patients.
Jonathan Sorof, Therapeutic Area Head – Immunology, Respiratory, Ophthalmology & Established Products at Roche, told Pharmafile why he believes the time has come for a tailored approach to severe asthma treatment, and explained why he thinks Roche is uniquely placed to deliver this.
He comments: “I think overall, there is a much better understanding of the potential targets we can leverage by knowing the specific cytokines, for example, these effector cytokines that are in the pathways for the cause of patients’ symptoms.
“So there has been an explosion of that science, and so with that knowledge, you can actually develop medicines that specifically target that cytokine and interfere with that pathogenesis that is causing asthma.
“I want to make sure that the medicine I pick is going to be the right medicine for that patient, because there is no one medicine that we currently believe will target all forms of asthma: there’s too many of them. But we do know there is a form of asthma called type 2 asthma, which is about half or more of the Spura?-controlled asthma patients, and that has a certain profile of pyromarkers that we are now better understanding, one of which is Periostin.
“We are developing tests for this within our diagnostics group, and the ability to have within one company, leading diagnostics capability and pharmaceutical drug development capability. We work very closely with our partners in diagnostics, and so we can develop at the same time the test that tells you who these patients are, what type of asthma they have and the medicines to take advantage of that knowledge to pick the right drug to target that patient.
Roche also revealed it is studying a humanised monoclonal antibody for severe uncontrolled asthma patients with type 2 inflammation. The antibody is designed to block the interleukin-13 protein that acts as a key inflammatory signal.
Encouraging treatment data to date show improved lung function and substantial reduction in asthma attacks. Roche says a diagnostic test based on this biomarker is currently being evaluated.
Dr Andrew Menzies-Gow, a consultant in respiratory medicine, spoke of his frustration at having to prescribe steroids to severe asthma patients in the knowledge that it can cause weight gain, and change the shape of the person’s face. Yet steroids remain the starting point for any treatment of severe asthma.
But he said the new developments made him “finally optimistic that I will rarely have to prescribe (steroid treatment) prednisolone.”
Teva presents reslizumab trial data
Teva presented results from a post hoc analysis of two pivotal Phase III clinical trials showing that treatment with reslizumab reduced clinical asthma exacerbations (CAEs) by 75 percent versus placebo in a subgroup of patients with late onset asthma (diagnosed at 40 years of age and older) with elevated blood eosinophils, and were inadequately controlled on inhaled corticosteroids (ICS).
Reslizumab is a humanized anti-interleukin-5 (IL-5) monoclonal antibody (mAb) for which Teva is seeking approval in the treatment of asthma in patients with elevated blood eosinophils who are inadequately controlled on an ICS-based regimen.
Data were pooled from two identical Phase III clinical trials (which were part of the BREATH clinical program) that comprised four placebo-controlled studies whose population of 1,700 adult and adolescent asthma patients (aged 12-75 years) had elevated blood eosinophils and symptoms that were inadequately controlled with ICS-based therapies.
“Asthma is a complex disease, and some phenotypes, such as late onset asthma with elevated blood eosinophils, can present particularly significant treatment challenges that are not adequately addressed by currently available therapies,” comments Michael Hayden, president of Global R&D and CSO at Teva. “Through the development of novel, targeted therapies, like reslizumab, Teva aims to provide safe and effective new treatment options to help more patients achieve improved asthma control.”
Tackling idiopathic pulmonary fibrosis
Pharma companies updated the Congress on their progress in the development of treatments for IPF: a fatal disease caused by irreversible, progressive scarring (fibrosis) of the lungs, which makes breathing difficult and prevents the heart, muscles and vital organs from receiving enough oxygen to function properly.
Treatment is complex, as variations in the rapidity of the disease’s progression are large, but in all cases the lungs will eventually harden and eventually stop functioning altogether. The outlook for patients post-diagnosis is poor- indeed worse than that of most cancers. In a recent study, only lung and pancreatic cancer were shown to have a worse survival outlook.
Half of IPF patients die within three years of diagnosis, and the five-year survival rate is approximately 20-40%. Approximately 100,000 people in the US and 110,000 people in Europe have IPF. The cause is currently unknown, and there is no cure. A limited number of patients with IPF undergo lung transplantation.
Roche presented new clinical data and abstracts on its Esbriet (pirfenidone) treatment at the Congress, showing a significant reduction in risk of death for patients who stayed on the treatment up to two years.
The medication’s mechanism of action is not fully understood, although it is believed to interfere with the production of transforming growth factor (TGF)-beta, a small protein in the body involved in how cells grow and produce scars (fibrosis), and tumour necrosis factor (TNF)-alpha, a small protein that is involved in inflammation.
The company said a pooled analysis from the Ascend and Capacity Phase III studies showed a 38% reduction in risk of death (p=0.0515) in IPF patients who took Esbriet for up to two years (120 weeks), compared with placebo.
Previously-reported data at one year showed the risk of mortality was reduced by 48% after treatment with Esbriet, a statistically significant result. The new data at 120 weeks show a strong trend in a reduced risk of death with long-term Esbriet treatment in IPF.
Also presented at the ERS, an ad-hoc analysis of the pooled Ascend and Capacity data showed patients who are hospitalised within the first six months of treatment saw their risk of disease progression (≥10% decline in lung function) or death reduced by more than two-thirds (relative difference = 72.2%) at one year by remaining on Esbriet treatment, compared with placebo.
Boehringer Ingelheim: new data demonstrate sustained long-term efficacy of OFEV in IPF treatment
On Tuesday 29, Boehringer Ingelheim reported from its INPULSIS-ON Phase III OFEV trial, confirming the efficacy and safety of its small molecule tyrosine kinase inhibitor OFEV (nintedanib) previously observed in prior INPULSIS trials.
Boehringer confirmed OFEV has a long-term effect on slowing disease progression and a manageable side effect profile in patients with idiopathic pulmonary fibrosis (IPF).
It is believed that nintedanib reduces disease progression in IPF and slows the decline in lung function by blocking the signalling pathways that are involved in fibrotic processes.
Boehringer emphasised the importance of the new data, given IPF’s status as a life-threatening and progressive disease which requires long-term treatment.
The INPULSIS-ON interim analysis showed that the change from baseline in forced vital capacity (FVC) at 48 weeks in patients continuing treatment with OFEV in the extension trial was comparable to that observed in the year-long INPULSIS trial, providing evidence of the medication’s long-term benefit.
- INPULSIS-ON- Key results
In INPULSIS-ON: -87 mL for all patients, -96,4 mL for patients continuing treatment with OFEV in the extension trial and -73,1 mL for patients initiating treatment with OFEV. - In INPULSIS (pooled data): -88,9 mL (OFEV) vs. -203,0 mL (placebo)
- No new safety signals were identified following long-term treatment with OFEV in INPULSIS-ON.
- The most frequent adverse events were gastrointestinal in nature with diarrhoea affecting 64% of patients but leading to drug discontinuation in only 5%.
The FDA approved OFEV for the treatment of IPF on 15 October 2014, and the European Commission likewise on 15 January 2015.
Chronic Obstructive Pulmonary Disease (COPD)
Boehringer presented new data showing an increase in FEV1 AUC0–12- a measure of lung function- with its Stiolto Respimat (tiotropium bromide and olodaterol) Inhalation Spray compared to a European formulation of a combination of long-acting beta agonist (LABA), salmeterol, and inhaled corticosteroid (ICS), fluticasone propionate.
Also presented at the ERS meeting was a post-hoc sub-analysis from the Ostemto 1&2 studies which investigated the effect of Stiolto Respimat on health-related quality of life, as reported by St. George’s Respiratory Questionnaire (SGRQ) total score, compared to tiotropium and placebo.
Joel Levy






