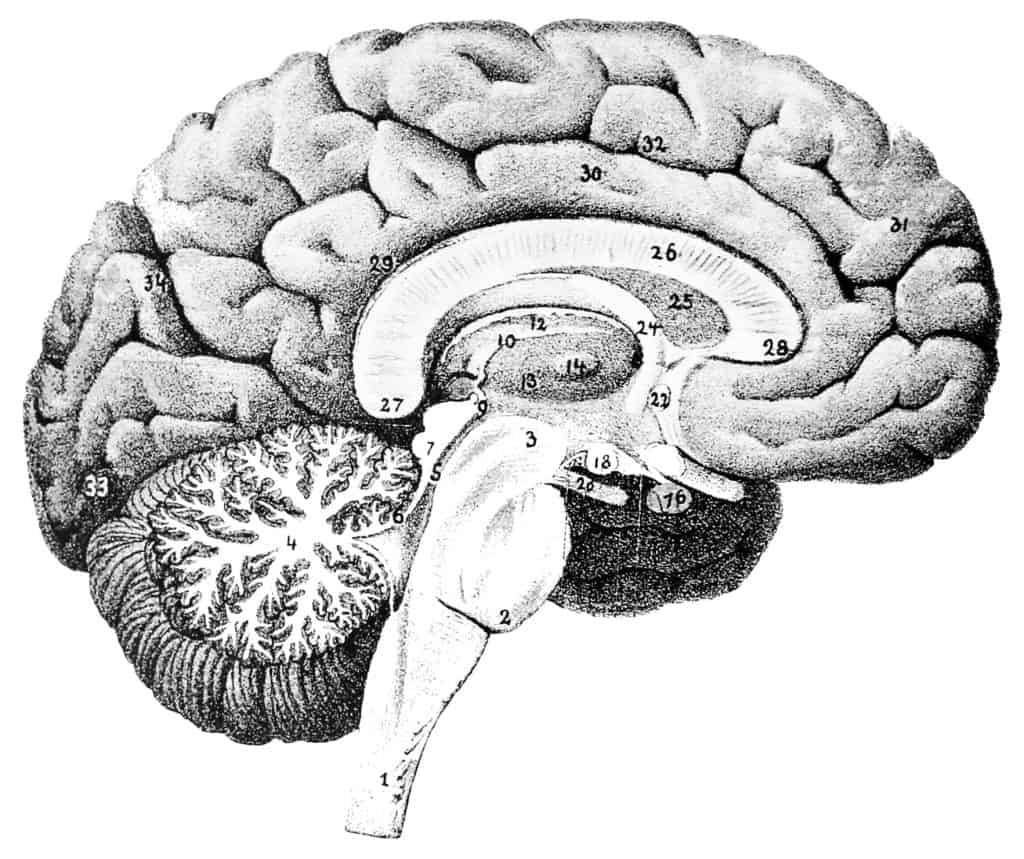
Drug slows brain shrinkage associated with progressive multiple sclerosis, study shows
pharmafile | August 30, 2018 | News story | Research and Development | MS, brain, ibudilast, neuroscience, research
The multiple sclerosis drug ibudilast, slowed brain shrinkages in patients with progressive MS, a study published in the New England Journal of Medicine has shown.
The study involving 255 participants with progressive multiple sclerosis revealed that while both patients in the control group and those taking ibudilast experienced brain shrinkage, those patients who took ibudilast experienced 2.5 millilitres of shrinkage less than those participants taking a placebo. This is specifically significant considering the whole adult brian has a volume of approximately 1,350 millilitres.
More specifically, the participants taking a placebo experienced 0.0009 units of atrophy more than those taking idubilast each year.
“The trial’s results are very encouraging and point towards a potential new therapy to help people with progressive MS,” said Dr Robert J Fox a neurologist at Cleveland Clinic in Ohio who led the study. “It also increased our understanding of advanced imaging techniques, so that future studies may require a smaller number of patients followed over a shorter period of time. This leads to increased efficiency of clinical research. These imaging methods may also be relevant to a host of other neurological disorders.”
Multiple sclerosis occurs when there is a breakdown of the fatty substance myelin that is wrapped around axons, the strands that carry messages between the brain and cells. When myelin breaks down communication between brain cells slows which thus leads to muscle weakness and problems with movement, balance, sensation and vision. Future research will test the extent to which reducing brain shrinkage affects the symptoms of MS and examine ibudilast’s ability to slow the progression of the disease.
“These findings provide a glimmer of hope for people with a form of multiple sclerosis that causes long-term disability but does not have many treatment options,” said Walter J. Koroshetz, M.D., director of the NINDS.
Louis Goss
Related Content
Johnson & Johnson submits FDA application for Caplyta to prevent schizophrenia relapse
Johnson & Johnson has submitted a supplemental New Drug Application to the US Food and …

Abbvie acquires Cerevel Therapeutics
Abbvie has announced the acquisition of Cerevel Therapeutics, strengthening its neuroscience pipeline. Cerevel has multiple …

Drug discovery and development partnership announced between Apollo Therapeutics and Oxford University
Portfolio therapeutics company Apollo Therapeutics has announced earlier this week that it will provide capital …






