Janssen to remove its Psoriasis 360 Facebook page
pharmafile | March 28, 2012 | News story | Medical Communications | Facebook, Janssen, PM Soc, Psoriasis 360
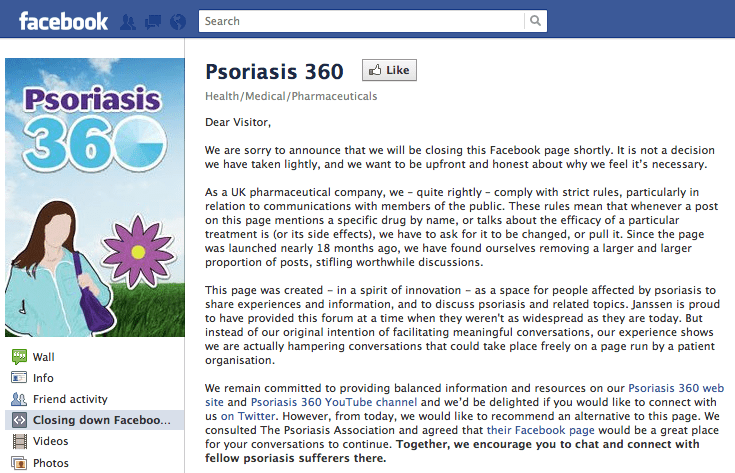
Janssen has decided to take down its award-winning Psoriasis 360 page on Facebook, citing an increasing amount of troublesome comments.
Since the page was launched nearly 18 months ago, Janssen says it has had to remove a larger and larger proportion of posts.
Janssen said: “Whenever a post on this page mentions a specific drug by name, or talks about the efficacy of a particular treatment is (or its side effects), we have to ask for it to be changed, or pull it.”
The firm said this has been ‘stifling worthwhile discussions’, and having to keep removing these posts had become too onerous for the firm.
All firms have been forced to make commenting available on their Facebook sites since August last year, although they are allowed to change or remove comments once they have been made.
Janssen markets its Stelara drug as a treatment option for adults with moderate to severe plaque psoriasis, and was using the site as a disease awareness campaign for the disease.
The firm said it remained committed to providing ‘balanced information and resources’ on its Psoriasis 360 web site, Psoriasis 360 YouTube channel, as well as its Twitter account – and stressed that these have not been affected by its Facebook decision.
The Psoriasis 360 project has been very successful, and Janssen picked up two awards for it from the PM Society’s Digital Media awards last year.
Psoriasis 360 wanted to use all aspects of social media to help with its disease awareness campaign about psoriasis, and its Facebook page was established to allow an online discussion about the disease.
Janssen said that it has consulted with the The Psoriasis Association, a charity it helps fund, and agreed that their Facebook page would be a good place for users of the Psoriasis 360 Facebook page to go to from now on.
The page is still viewable and the public can make comments, but Janssen plans to close it down completely in the coming months.
Ben Adams is the reporter for Pharmafocus and InPharm.com and manages the DigiBlog site. He can be contacted via: email or Twitter.
Related Content

Janssen’s study for nipocalimab as indicated for EOS-HDFN published in The New England Journal of Medicine
Johnson & Johnson company Janssen has announced that the results from its phase 2 trial …

GSK enters agreement for license for JNJ-3089 for development of bepirovirsen
GSK and Arrowhead Pharmaceuticals have announced that they have come to an agreement with Janssen …

Janssen and Sanofi enter agreement for potential vaccine programme
Janssen Pharmaceuticals, a Johnson & Johnson company, has announced a development and commercialisation agreement with …






