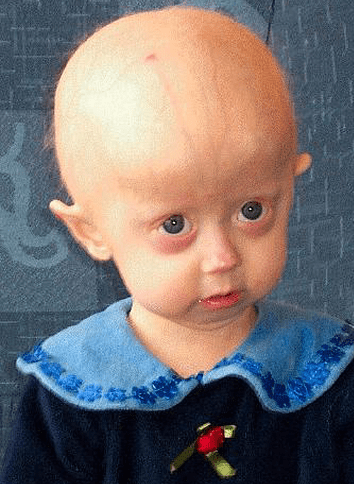
FDA approves first drug to treat rare rapid ageing condition progeria
pharmafile | November 26, 2020 | News story | Business Services |
The FDA has approved the first drug to treat the rare genetic disorder progeria, a rare condition which causes cells to age rapidly and prematurely, leading children to appear older before they reach maturity. Most people with this condition die in their early teens from heart failure, heart attack or stroke.
The drug Zokinvy, developed by Eiger Biopharmaceuticals in a tablet formulation, blocks some of the progerin production that lowers the amount that accumulates in the children’s cells. It does not fully block production and cause fatigue and vomiting.
The trial that led to its approval was a study involving 62 children with the condition. It increased the life span of those with the condition by three months on average during the first three years of treatment, compared with data from another study that looked at children who did not receive the treatment. Children who continued to receive Zokinvy for up to 11 years saw their average lifespan lengthen by about 2.5 years.
Monica Kleinman, a paediatric critical care doctor at Boston Children’s Hospital who was involved with the clinical trials, remarked: “This is not a cure. We’ve hopefully extended the life span by slowing the pace of the disease.”
The condition is so rare it is estimated that only between 350 and 400 children across the world live with the condition.
Conor Kavanagh








