NHS England to supply HIV prevention drug PrEP with £16m government investment
pharmafile | March 16, 2020 | News story | Manufacturing and Production, Sales and Marketing | NHS, NHS England, PrEP HIV, PrEP< HIV, UK, pharma
NHS England is set to fund the availability of pre-exposure prophylaxis (PrEP) for the prevention of HIV for local authorities across the country from April, the UK’s Department of Health and Social Care has revealed.
The drive comprises an investment of £16 million to local authorities, including sexual health clinics, throughout 2020 and 2021. The preventative measure will be made available to “anyone who is at a high risk of contracting HIV” and follows the UK Government’s commitment in 2019 to end HIV transmission by 2030.
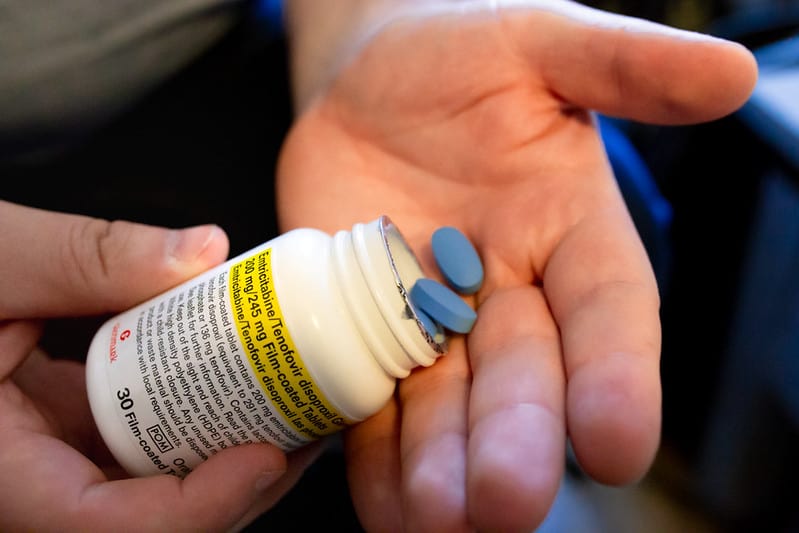
PrEP has demonstrated a 99% reduction in the risk of contracting HIV through intercourse in clinical trials when taken daily.
Until now, the drug has only been available to patients via the PrEP impact trial, a three-year study of over 25,000 people living with HIV. Specifically, the trial utilises Mylan’s biosimilar version of Gilead’s Truvada (emtricitabine/tenofovir disoproxil). This newly announced funding also covers the continued treatment of these patients once they have finished the study following its closure in October later this year.
“While it is encouraging to see HIV transmissions continue to fall across the UK, I am determined to do more, and end HIV transmission,” commented Health and Social Care Secretary Matt Hancock on announcement of the news. “So we are rolling out PrEP and making it available across the country – with evidence showing it almost completely eradicates the chances of getting HIV. This will benefit tens of thousands of people’s lives, and drive us towards our ambition of zero HIV transmissions in this decade.”
Matt Fellows
Related Content

NICE recommends migraine treatment for NHS use
The National Institute for Health and Care Excellence (NICE) has shared draft guidance recommending AbbVie’s …

Novo Nordisk launches Wegovy in the UK
Novo Nordisk has today announced that Wegovy (semaglutide injection) is now available in the UK …

FDA approves IMIDEX’s AI-powered device VisiRad XR
The technological pharmaceutical company IMIDEX has been granted clearance from the US Food and Drug …








