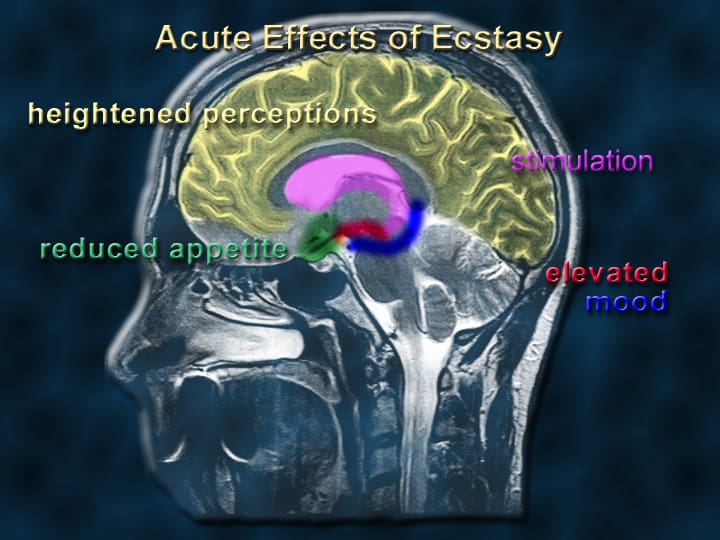
Patients to recieve MDMA-assisted psychotherapy on ‘compassionate’ grounds in Israel
pharmafile | February 28, 2019 | News story | Research and Development | MDMA, PTSD, drugs, mental health, psychedelics
The Israeli Health Ministry has approved the compassionate use of MDMA-assisted psychotherapy for 50 patients with post-traumatic stress disorder (PTSD).
The Israeli government currently classify MDMA as a ‘dangerous drug’ While recreational use of MDMA is illegal, the government allow use of MDMA in clinical trials.
However compassionate use allows drugs that are still in development to be made available to patients outside of a clinical trial due to lack of effective alternatives.
“The ministry is taking this seriously and with appropriate caution, an in-depth investigation has been carried out. There is a considerable population in Israel of people suffering from PTSD that is resistant to other treatment,” Bella Ben-Gershon of Israel’s Ministry of Health said to Haaretz.
The Multidisciplinary Association for Psychedelic Studies (MAPS) initiated Phase 3 clinical trials of MDMA-assisted psychotherapy in Israel on 5 February 2019. The therapy was granted ‘breakthrough therapy’ status by the US FDA in 2017.
If all goes to plan MDMA-assisted psychotherapy will gain FDA approval by 2021.
Patients living with PTSD will be eligible to receive treatment at four sites throughout Israel, including Rambam Medical Center in Haifa and psychiatric hospitals in Be’er Yaakov, Lev Hasharon, and Be’er Sheva.
Louis Goss
Related Content

Intra-Cellular Therapies shares phase 3 results for major depressive disorder therapy
Intra-Cellular Therapies has announced positive topline results from Study 501, which assessed lumateperone 42mg as …

Karuna Therapeutics submits NDA to FDA for schizophrenia treatment
Karuna Therapeutics has announced that it has submitted a New Drug Application (NDA) to the …

PharmAla receives MDMA research grant from Ontario Centre of Innovation
Canadian biotechnology company PharmAla has announced that it has been awarded a research grant from …








